Calculate the number of moles of c nc – In the realm of chemistry, understanding the concept of moles is crucial for comprehending the behavior and interactions of substances. This guide delves into the intricacies of calculating the number of moles of carbon monoxide (CO), a fundamental aspect of quantitative chemical analysis.
The mole, defined as the amount of substance that contains exactly 6.02214076×10 23elementary entities, serves as the cornerstone of mole calculations. This guide will explore the formula for calculating moles, the determination of molar mass, and the practical applications of mole calculations in chemistry.
Definition of Moles

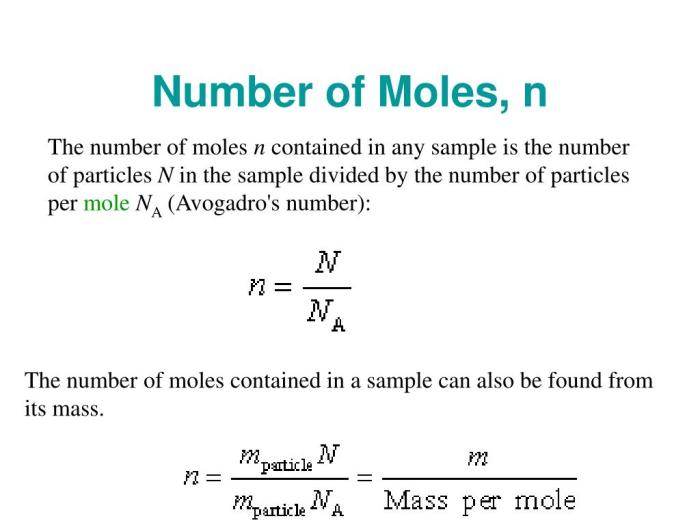
In chemistry, a mole is a fundamental unit of measurement used to quantify the amount of a substance. It is defined as the amount of a substance that contains exactly 6.02214076 x 10^23 elementary entities. These entities can be atoms, molecules, ions, or electrons, depending on the substance being measured.
The concept of the mole is closely related to Avogadro’s number, which represents the number of elementary entities present in one mole of a substance. Avogadro’s number is an extremely large number, approximately 6.022 x 10^23. This means that one mole of a substance contains a vast number of individual particles, making it a convenient unit for measuring large quantities of chemical substances.
Calculating Moles of Carbon Monoxide (CO)
To determine the number of moles of carbon monoxide (CO) present in a given sample, we employ the formula:“`moles = mass (g) / molar mass (g/mol)“`The molar mass of a compound represents the mass of one mole of that compound.
For CO, the molar mass is 28.01 g/mol, which is the sum of the atomic masses of carbon (12.01 g/mol) and oxygen (16.00 g/mol).
Example Calculations
Let’s demonstrate the calculation of moles of CO for different masses:
- Mass = 14.01 g“` moles = 14.01 g / 28.01 g/mol = 0.5 moles “`
- Mass = 56.02 g“` moles = 56.02 g / 28.01 g/mol = 2.0 moles “`
- Mass = 112.04 g“` moles = 112.04 g / 28.01 g/mol = 4.0 moles “`
By utilizing the formula and molar mass, we can accurately determine the number of moles of CO present in various samples.
Applications of Mole Calculations

Mole calculations are indispensable tools in chemistry, enabling scientists to determine the quantities of reactants and products involved in chemical reactions, as well as to calculate various properties of chemical substances.
One of the most important applications of mole calculations is in stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions. By using mole ratios derived from balanced chemical equations, chemists can predict the exact amounts of reactants and products that will be involved in a given reaction.
Examples of Applications
- Determining reactant and product quantities:Mole calculations allow chemists to determine the precise amounts of reactants needed to produce a desired quantity of product or the amount of product that can be obtained from a given quantity of reactants.
- Calculating limiting reactants:In reactions involving multiple reactants, mole calculations help identify the limiting reactant, which is the reactant that is entirely consumed, thereby limiting the amount of product that can be formed.
- Predicting reaction yields:Mole calculations enable chemists to predict the theoretical yield of a reaction, which is the maximum amount of product that can be obtained under ideal conditions.
- Determining the concentration of solutions:Mole calculations are crucial for determining the concentration of solutions, which is expressed in terms of molarity (moles of solute per liter of solution).
- Calculating gas volumes:Mole calculations can be used to determine the volume of a gas at a given temperature and pressure, using the ideal gas law (PV = nRT).
Factors Affecting Mole Calculations: Calculate The Number Of Moles Of C Nc
The number of moles in a sample can be affected by temperature and pressure. These factors can be accounted for in mole calculations using the ideal gas law.
Temperature
As temperature increases, the number of moles in a sample increases. This is because the particles in the sample move faster at higher temperatures, which means they have more energy and are more likely to escape from the sample.
The following equation can be used to calculate the number of moles in a sample at a given temperature:
“`n = PV/RT“`where:* n is the number of moles in the sample
- P is the pressure of the sample
- V is the volume of the sample
- R is the ideal gas constant
- T is the temperature of the sample
Pressure, Calculate the number of moles of c nc
As pressure increases, the number of moles in a sample decreases. This is because the particles in the sample are forced closer together at higher pressures, which means they have less energy and are less likely to escape from the sample.
The following equation can be used to calculate the number of moles in a sample at a given pressure:
“`n = PV/RT“`where:* n is the number of moles in the sample
- P is the pressure of the sample
- V is the volume of the sample
- R is the ideal gas constant
- T is the temperature of the sample
Table Summarizing the Effects of Temperature and Pressure
| Factor | Effect on Number of Moles |
|---|---|
| Temperature | Increases |
| Pressure | Decreases |
Error Analysis in Mole Calculations

Errors in mole calculations can arise from various sources, leading to inaccurate results. It is crucial to identify and minimize these errors to ensure reliable outcomes.
Common sources of error include:
- Inaccurate measurements of mass or volume
- Incorrect formulas or unit conversions
- Calculation mistakes
Minimizing Errors
To minimize errors, it is essential to:
- Use calibrated and precise measuring instruments
- Double-check measurements and calculations
- Pay attention to units and conversions
- Seek assistance from a calculator or spreadsheet
Error Analysis Process
The following flowchart Artikels the error analysis process:
- Identify potential sources of error
- Estimate the magnitude of each error
- Determine the cumulative effect of errors
- Apply error propagation formulas to calculate the uncertainty in the final result
Essential Questionnaire
What is the formula for calculating moles?
Moles = Mass (g) / Molar Mass (g/mol)
How do I determine the molar mass of CO?
Molar mass of CO = Atomic mass of carbon (12.01 g/mol) + Atomic mass of oxygen (16.00 g/mol) = 28.01 g/mol
What are some applications of mole calculations?
Mole calculations are used to determine reactant and product quantities in chemical reactions, calculate gas volumes, and analyze the composition of substances.